-
Inari Medical’s FlowTriever System Receives FDA 510(k) Clearance for Treatment of Right Atrial Clot in Transit
Source: Nasdaq GlobeNewswire / 08 Jan 2021 08:00:00 America/New_York
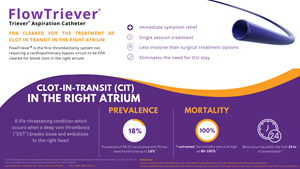
IRVINE, Calif., Jan. 08, 2021 (GLOBE NEWSWIRE) -- Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced U.S. Food and Drug Administration (“FDA”) 510(k) clearance of the FlowTriever system for the treatment of clot in transit (“CIT”) in the right atrium.
CIT is a life-threatening condition which occurs when a deep vein thrombosis (“DVT”) breaks loose and embolizes to the right heart. 25,000 patients are diagnosed with right atrial CIT in the United States annually, and the condition is associated with a mortality rate of over 80% if left untreated. FlowTriever is the first thrombectomy system not requiring a cardiopulmonary bypass circuit to be FDA cleared for blood clots in the right atrium.
Interventional cardiologists Dr. Gautam Kumar and Dr. Rajesh Sachdeva recently co-authored a case series on the successful FlowTriever right atrial CIT experience they have had respectively at Emory and Morehouse Schools of Medicine and their affiliated hospitals, in Catheterization and Cardiovascular Intervention, the official journal of the Society for Cardiovascular Angiography and Interventions (“SCAI”). “Right atrial CIT is a serious condition requiring urgent intervention, yet no single best treatment modality has been established,” said Dr. Kumar. “Intervention has traditionally exposed critically ill patients to the risks of open-heart surgery or thrombolytic drugs, or required the setup of complex bypass circuits by specialized perfusion staff in an operating room under general anesthesia. FlowTriever offers an exciting new treatment option to safely remove clot from the right atrium in a short, single session procedure without general anesthesia while avoiding the bleeding risks of thrombolytics.”
“This expanded indication for FlowTriever is the latest testament to Inari’s comprehensive and long-term commitment to the care of venous thromboembolism (“VTE”) patients,” said Bill Hoffman, Inari’s Chief Executive Officer. “We remain committed to revolutionizing VTE treatment with devices that remove large clot volume from large vessels, now including within the heart, while completely eliminating lytics and their consequent cost, ICU stay, and bleeding complications.”
About Inari Medical, Inc.
Inari Medical, Inc. is a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases. Inari has developed two minimally-invasive, novel catheter-based mechanical thrombectomy devices that are designed to remove large clots from large vessels and eliminate the need for thrombolytic drugs. The company purpose-built its products for the specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism, or VTE: deep vein thrombosis and pulmonary embolism. The ClotTriever system is 510(k)-cleared by the FDA and CE Mark approved for the treatment of deep vein thrombosis. The FlowTriever system is 510(k)-cleared by the FDA for the treatment of pulmonary embolism and clot in transit in the right atrium.Investor Contact:
Westwicke Partners
Caroline Corner
Phone +1-415-202-5678
caroline.corner@westwicke.comA photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d6804fd5-394f-48fa-8e9a-979e9dababbe